- Exciting News! We’ve partnered with Affirm and CareCredit to offer flexible payment plans, making early cancer detection even more accessible.
The Science of Early Cancer Detection
TruView OncoScreen® uses rapid in situ hybridization and DNA fluorescent probes to detect chromosomal changes linked to cancer — even at the earliest stages — enabling fast, affordable, and comprehensive screening without invasive procedures.
All Human Cancers
Comprehensive testing for every type of cancer with advanced genomic analysis
Results in 24 Hours
Get your results the next day, no weeks of waiting, no anxiety limbo
97.7% Accuracy
Industry-leading precision with validated clinical-grade testing protocols
For Providers
TruView OncoScreen®’s
Advanced Cancer Screening
Technology.
Detecting cancer can be challenging due to limited existing screening tests, long turnaround times, high costs, and invasive procedures.TruView OncoScreen® ‘s innovative technology allows for early detection, even at stage -1, and provides crucial information for guiding further imaging tests with our detailed Oncosome count.
TruView OncoScreen® ‘s advanced cancer screening technology utilizes rapid in situ hybridization and DNA fluorescent probes, offering comprehensive cancer screening with high accuracy. Our approach includes a rapid SITU Hybridization Platform, DNA Fluorescent Probes for ploidy detection, and inexpensive density gradient and low sequential centrifugation, which enable comprehensive screening for aneuploidy of all chromosomes.
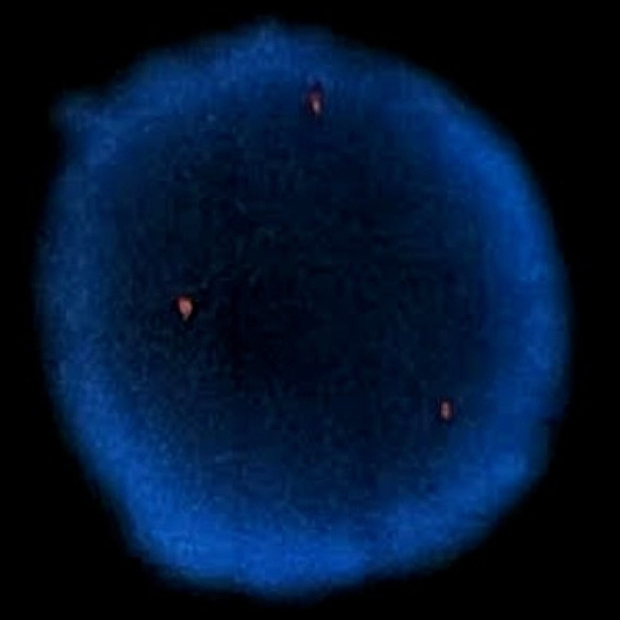
The Science Behind TruView OncoScreen®
OncoSure leverages cutting-edge technology to redefine cancer diagnostics.
Oncosomes: Cancer Specific Markers
Oncosomes are microscopic vesicles unique to cancer cells. By isolating and analyzing these, OncoSure achieves high specificity and sensitivity in early cancer detection.
Advanced Hybridization Technology
Utilizing rapid in situ hybridization and DNA fluorescent probes, OncoSure offers comprehensive screening for aneuploidy across all chromosomes, ensuring thorough and accurate results.
Expert Leadership: Dr. Ramesh Babu
Developed under the guidance of Dr. Ramesh Babu, a pioneer in cancer research with extensive experience in oncology and molecular diagnostics, OncoSure reflects a commitment to advancing early detection methods for improved patient outcomes.
CLINICAL STUDY
Download TruView OncoScreen® 's Cancer Clinical Study Report
Download our comprehensive whitepaper to learn more about the latest advancements in cancer testing technology and how TruView OncoScreen® is paving the way for faster, more accurate cancer detection.

Our Expert Team
World-Class Scientists & Laboratory Professionals
Our team of board-certified physicians, molecular scientists, and laboratory professionals brings decades of experience in molecular diagnostics, pathology, and precision medicine to deliver the highest quality testing services.
Double Board-Certified
Dr. Phoutthasone Thirakul, MD
Laboratory Director
Pathology & Forensic Medicine
Education:
University of California, San Diego (MD)
University of South Florida (Fellowship)
Areas of Expertise:
Anatomic & Clinical Pathology
Forensic Pathology
Laboratory Operations
Oversees all clinical and operational aspects of the laboratory, ensuring compliance with regulatory standards and maintaining the highest levels of diagnostic accuracy.
Advanced Certifications
Jahmar Hudson
Chief Scientific Officer & Technical Supervisor
Molecular Diagnostics & Pharmacogenomics
Education:
University of Cincinnati (MS Pharmacogenomics)
Nova Southeastern University (PhD in progress)
Areas of Expertise:
Molecular Diagnostics
Pharmacogenomics
Quality Assurance
Provides scientific leadership and technical oversight of molecular diagnostics, focusing on assay development, validation, and regulatory compliance.
CLIA Supervisor
Stephanie Altidort
General Supervisor, Molecular Pathology
Molecular Diagnostics & Genomics
Education:
Florida Atlantic University (BS Neuroscience)
Areas of Expertise:
High-Complexity Testing
Quality Control
Staff Management
Supervises high-complexity molecular testing procedures and ensures compliance with CLIA regulatory standards and laboratory accreditation requirements.
Clinical Specialist
Stephanie Lucce
Molecular Technologist
Biotechnology & Molecular Pathology
Education:
Miami Dade College (BS Biotechnology)
Areas of Expertise:
PCR Techniques
Nucleic Acid Analysis
Genetic Testing
Executes high-complexity molecular assays including nucleic acid extraction, PCR amplification, and genetic analysis for infectious diseases and genetic conditions.
20+ Years Experience
William Wysock
Molecular Scientist
Validation & Process Development
Education:
Catholic University of America (MS Biology)
Loyola University (BA Writing)
Areas of Expertise:
Method Validation
NGS Technologies
Six Sigma Quality
Oversees validation processes and complex technical inquiries, with extensive experience in molecular methodologies and regulatory compliance.



